Vascular
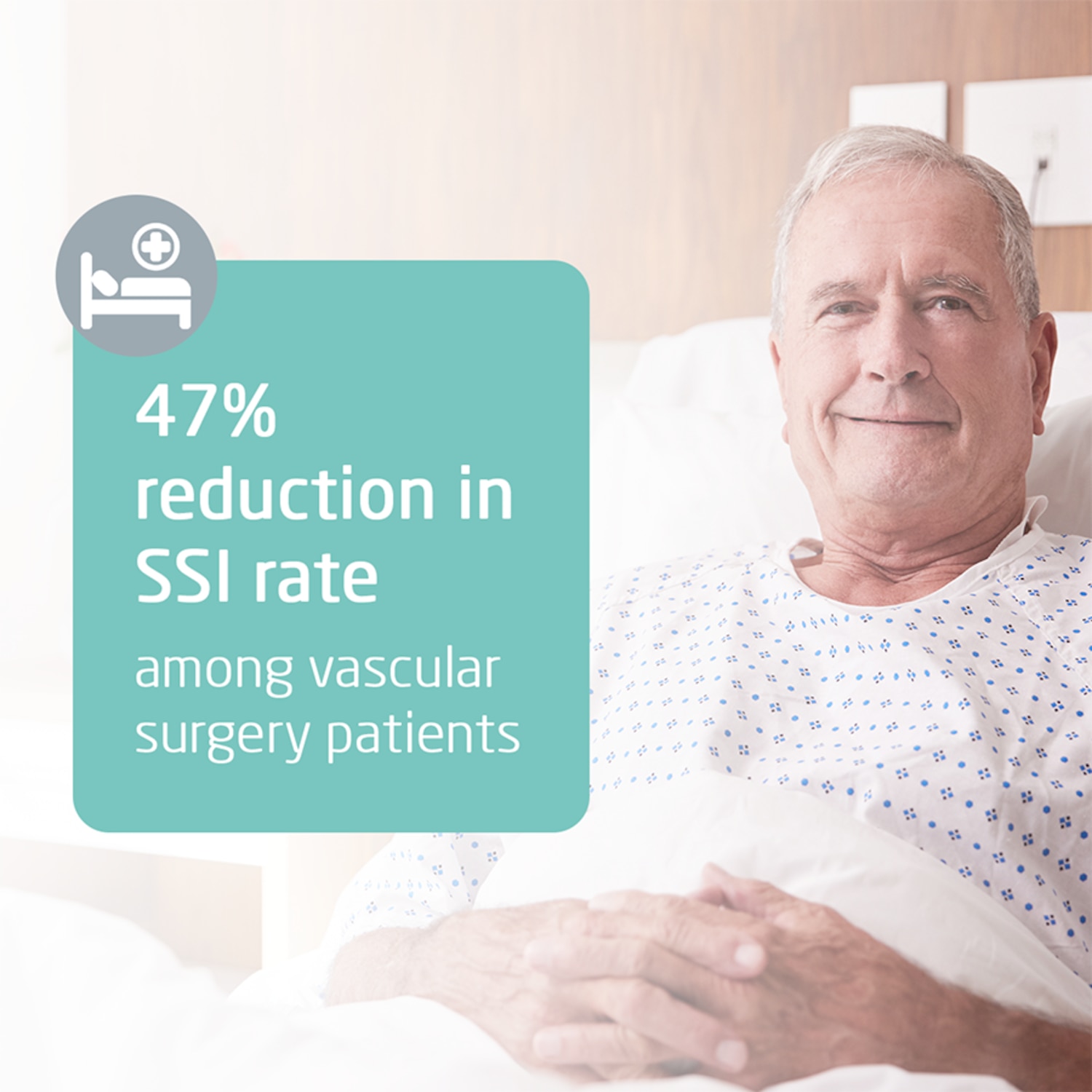
SSIs are the third most commonly reported type of healthcare-acquired infection and the most costly1. They place a significant impact on patient welfare2 as well as presenting a heavy financial burden for the NHS3.
Leukomed® Sorbact® is an innovative post-operative film dressing with a purely physical mode of action, used to prevent surgical site infection (SSI) in closed surgical wounds. Following rigorous assessment by leading clinical and health economic experts during the NICE Medical Technology appraisal process it is proposed that Leukomed Sorbact should be considered as an option for preventing surgical site infection (SSI) in wounds with low to moderate exudate after caesarean section and vascular surgery. It should be used as part of usual measures to help reduce the risk of surgical site infection*.
Get your clinical evidence and sample pack here